
![[image]](https://WFMJ.images.worldnow.com/images/18361370_G.jpg)
A pharmaceutical company is recalling some heat wraps sold at Walmart and other retailers that could become too hot and cause burns.
Pfizer Consumer Healthcare has informed the U.S. Food and Drug Administration that it is recalling one lot of ThermaCare® Back Pain Therapy HeatWraps that offer up to 16HR pain relief.
The company says the recalled heat wraps may include cells that have a higher temperature than specified.
The use of a wrap with a cell with increased temperature poses a potential risk of skin injuries such as burns/blisters and/or skin irritation on the wrap applied area.
Consumers should contact their healthcare provider if they have experienced any problems that may be related to using this product.
The ThermaCare® Back Pain Therapy HeatWraps, up to 16HR pain relief, master lot impacted is S97473, UPC 305733010037 (Lower Back and Hip Therapy, two count carton) and was used in the manufacturing of 17 retail display case sub-lots.
The lot number can be found on the side of ThermaCare cartons and the back of ThermaCare pouches.
ThermaCare® HeatWraps Retail Display Information:
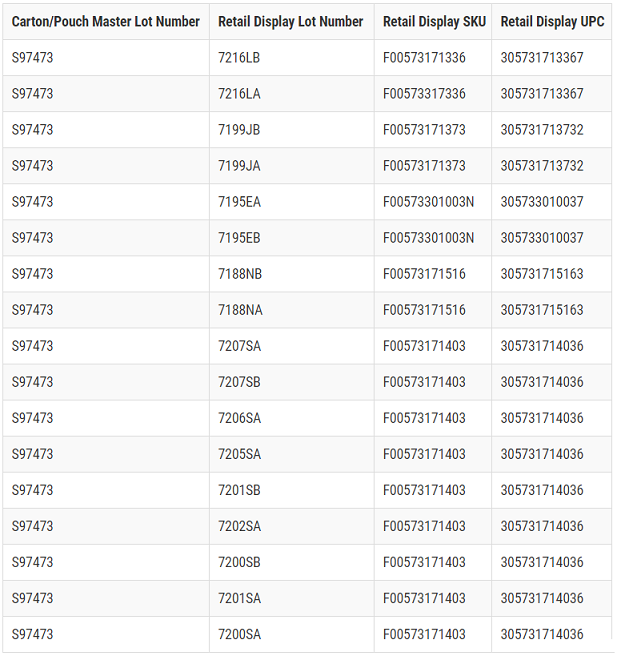
This master lot and display case sub lots were distributed nationwide to retailers, wholesalers, and distributors in the United States and Puerto Rico from June 2017 through March 2018.
Pfizer Consumer Healthcare is removing the recalled heat wraps from store shelves and asking people who purchased them to discontinue use of the product, record the lot number, throw the product away in its entirety without opening the foil pouch, and to contact the Pfizer Consumer Healthcare Information Line at 1-800-323-3383 (Mon-Fri, 9 am-5 pm EST) for replacement or reimbursement.
Wholesalers, distributors, and retailers with an existing inventory of the lot being recalled are being advised to stop use and distribution and quarantine the product immediately.
Retailers that have already distributed the recalled product are being told to notify any accounts or additional locations which may have received the recalled product.
For retailer instructions on returning the product or additional assistance, call Stericycle at 1-800-805-3093 between the hours of 8 am to 5 pm ET, Monday through Friday.
If consumers have questions regarding this recall or to report an adverse event or product complaint, contact the Pfizer Consumer Healthcare Information Line at 1-800-323-3383 (Mon-Fri, 9am-5pm EST).
Adverse reactions or quality problems experienced with the use of this product may also be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report
Online: www.fda.gov/medwatch/report.htm
Regular Mail or Fax: Download the form at www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
The product label recommends the user to stop use or wear a layer of clothing if the wrap feels too hot to prevent skin injuries.
ThermaCare® Back Pain Therapy HeatWraps provide heat therapy for temporary relief of minor muscular and joint aches and pains associated with overexertion, strains, sprains, and arthritis.