Recall issued for 'unsanitary' eye drops sold at Target, Walmart, CVS, Rite Aid

WASHINGTON - The Food and Drug Administration has announced the recall of potentially harmful eye drop products sold at national retailers including Target, Rite Aid, CVS, and Walmart.
The products are being recalled due to potential safety concerns after FDA investigators found insanitary conditions.
Kilitch Healthcare India Limited is recalling eye drops due to a potential risk of eye infections or related harm. These products are intended to be sterile. Ophthalmic drug products pose a potentially heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses.
The recall involves products listed in the table below with all lots within expiry with expiration dates ranging from November 2023 to September 2025.
To date, Kilitch Healthcare India Limited says it has not received any reports of adverse events related to this recall.
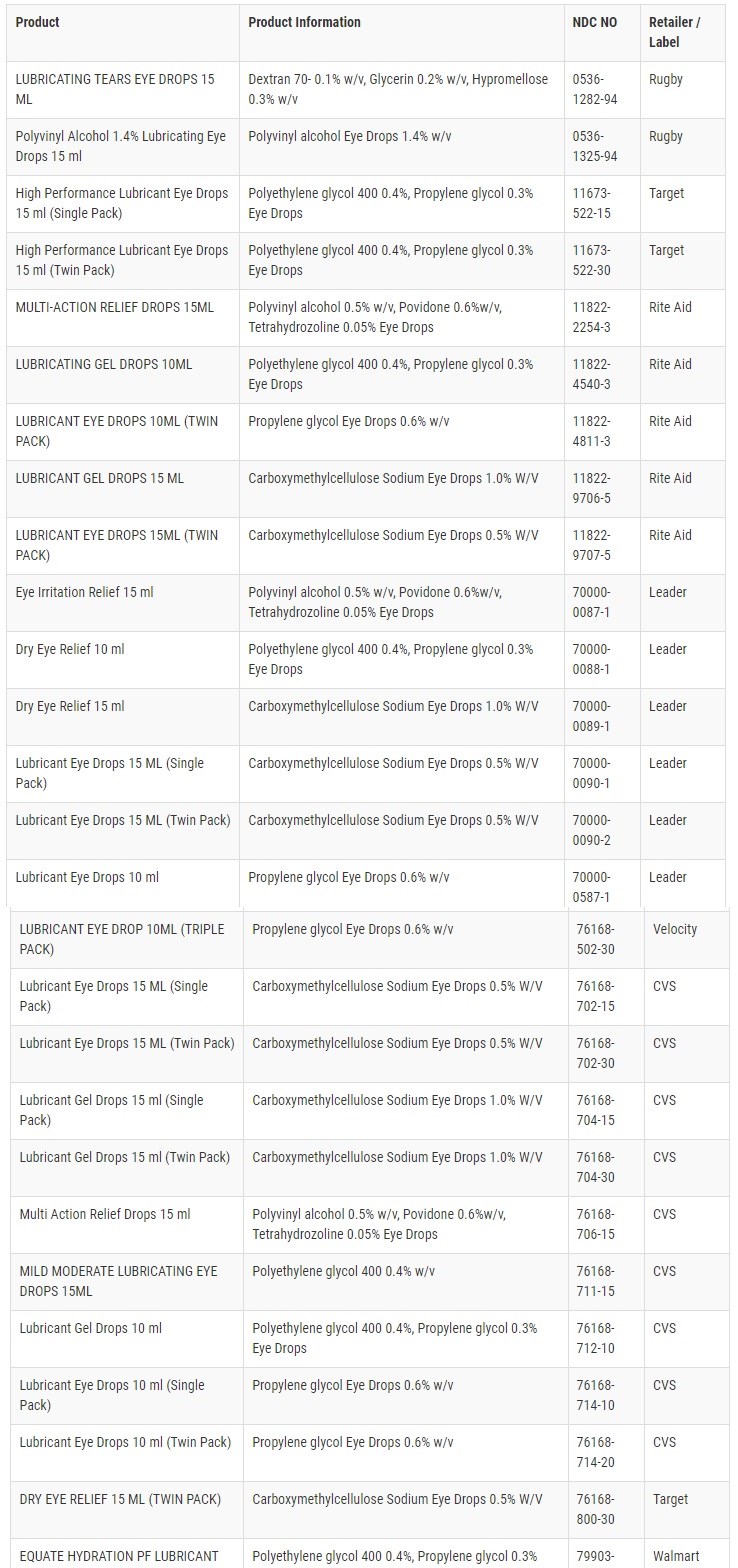
These products were distributed nationwide to wholesalers, retailers, and via the product distributor, Velocity Pharma LLC.
Consumers, distributors, and retailers that have any product that is being recalled should cease distribution. Consumers should stop using the recalled eye drops and may return any of the above-listed products to the place of purchase.
Anyone who has experienced problems that may be related to taking or using the product should contact their physician or healthcare provider.












